 |
| Protein bound Nano Particles Quantitative bioassays for Total and Unbounded fraction |
|---|
| Protein-bound paclitaxel (nanoparticle albumin–bound) is an injectable formulation of paclitaxel, which is used to treat breast cancer, lung cancer and pancreatic cancer among others. Paclitaxel destroys cancer cells by preventing the normal breakdown of microtubules during cell division. In this formulation, paclitaxel is bonded to albumin as a delivery vehicle.
|
| A new formulation called Abraxane an albumin bounded form of Paclitaxel is introduced by Abraxis Bioscience, Inc is the first successful example of nab technology-based drug delivery, and consists of Paclitaxel protein-bound particles for inject able suspension (albumin bound). Abraxane or nab-Paclitaxel, is a Cremophore-free, albumin-bound 130-nm particle form of Paclitaxel. The formulation is approved by the FDA in January 2005 for the treatment of breast cancer after a failure of combination chemotherapy for metastatic disease.
|
| Abraxane consists of particles of Paclitaxel in the nanometer-size range, stabilized with human albumin. The Paclitaxel and albumin are not covalently linked but rather associated through hydrophobic interactions. The particles of Paclitaxel are in a non-crystalline, amorphous, readily bioavailable state, allowing for rapid drug release from the particles following intravenous administration. Once injected into circulation, Paclitaxel nanoparticle quickly dissolve into smaller albumin-Paclitaxel complexes whose size is virtually identical to that of endogenous albumin molecules in blood. Thus, the albumin-Paclitaxel complexes are fully capable of utilizing the natural albumin pathways | 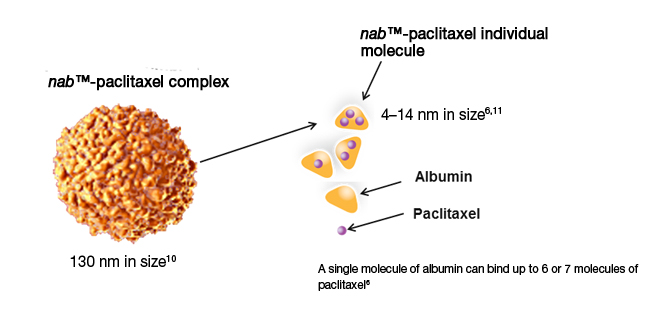 |
|
| Method development for Bioanalysis of this drug poses challenges in precise measurement of free and total drugs in the biological fluid. Veeda With its team of experienced scientist can provide you solution with efficient and precise methods. | 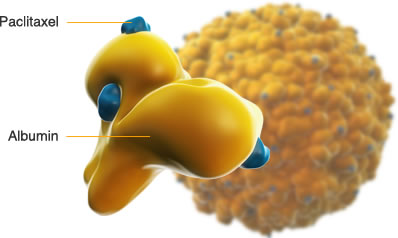 |
|
Main challenges for Albumin Bound Nano particle Bioassays :
- Precise method for quantitation of free (unbound fraction) and total drugs.
- Experienced Method development team to handle complex assay methodologies
- Sample handling to achieve the regulatory requirement and validation as per guidance for albumin bound drugs and unbound fraction of drug.
- Method reproducibility and turnaround time for Bioassay.
- Dissociation and accurate measurement of Albumin bound Paclitaxel.
|
Our Capabilities :
- Team having experience and validated method for the Total paclitaxel and unbound paclitaxel.
- Trained bioanalysts to handle complex methods.
- Successfully separate and Quantify unbound fraction of drug.
- Developed Suitable extraction methodology for the dissociation of Albumin bound Paclitaxel.
- Method was successfully employed for the measurement of free (unbound fraction) and total paclitexal in patient samples using test formulation, and reference formulation (ABRAXANE®) 5mg/mL(260mg/m2).
- Incurred sample reanalysis performed and >93% samples found within predefined acceptance limit which proves the reproducibility of method.
|
| |
Veeda Advantage
- 100% data review by Bio-analytical Quality Monitors
- State of art Bio analytical Lab equipped with highend sensitive equipment’s to achieve the required LLOQ.
- Trained Bio analysts to handle complex sample processing
- Proven regulatory track record with 11 USFDA, 5 European, 4 WHO &11 ANVISA audits
- Trusted CRO partner to 10 of the world’s top 15 Global Pharmaceutical Companies
- Bio-analytical Unit with more than 590 validated assays in its library of compounds including 36 NCE methods, 20 more under development.
|
|
| VEEDA CLINICAL RESEARCH PVT. LTD. Vedant Complex, Beside YMCA club S.G.Highway, Vejalpur,Ahmedabad-380051,Gujarat India Phone: +91 79 3001 3000 | Fax: +91 79 3001 3010 | Email: info@veedacr.com
Website: www.veedacr.com
|
 |



